Benefits of Bysglycinate

Many people have questions about bysglycinate chelated minerals. And rightfully so, it can be challenging to find quality information about it online. We prefer bisglycinated chelated minerals in our formulas over other forms for several reasons, which this article outlines.
Many of our staple supplements contain bisglycinate chelated minerals such as copper-, boron-, and magnesium bisglycinate. We believe this form of mineral to be superior regarding bioavailability.
Mineral Biochemistry
Minerals like magnesium, copper, and boron have free electrons in their outer shell. These are called “valence electrons,” and they are what participate in forming bonds and reacting with other elements or molecules.
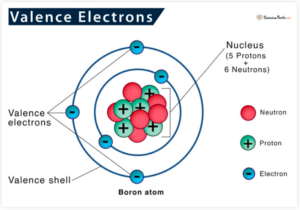
https://www.sciencefacts.net/valence-electrons.html IMAGE LINK
Atoms prefer to have a full outer or valence shell consisting of 4 electrons. To achieve this, they often share other electrons with other atoms. This makes them more stable
Some elements become very unstable when they lose their valence electrons. Antioxidants donate their free electron(s) to reduce other oxidant molecules. Oxidants themselves get their name from the fact that they steal electrons, effectively oxidizing them. This is the chemical reaction behind the concept of “oxidative stress.”
Some minerals are more stable when they’ve lost their valence electrons because the inner shells are filled. Such is the case when ferric iron (Fe3+) is reduced to ferrous iron (Fe2+). The 3+ indicates that iron has lost 3 of its valence electrons, giving it a net positive charge. Reducing it to Fe2+ means that it’s gained an electron, giving it 2 electrons in its valence shell. Because atoms want to fill this shell, Fe2+ is now more likely to steal 2 electrons (usually from H2O2 via the Fenton reaction). This can result in tissue-damaging hydroxyl radicals that cause oxidative stress (1).
Mineral Absorption
The natural state of elements is to exist in their least reactive forms. But when transporting in and out of cells, minerals often need to donate or gain an electron prior to entering.
For example, copper (Cu) from foods is predominantly in the Cu2+ state, meaning it’s lost 2 electrons from its outer shell. For copper to be absorbed in the gut, it must first gain an electron, thereby “reducing” its state to Cu1+ (2, 3). When copper is consumed from a whole food source, the extra electron is donated by other molecules like vitamin C.
This absorption action happens via specific transport receptors on the brush border cells of the interstitial tract. Absorption efficiency is often governed by the level of said mineral already present in the blood. Additionally, various minerals use the same transporters to enter the cells; thus, they must compete with each other for absorption (3).
Some components in plant foods can also inhibit mineral absorption. Phytates, tannins, and saponins can bind to minerals and block their absorption (4). These compounds are easily degraded with heat from cooking and do not pose a significant health threat. However, they are worth noting in the case of minerals present in lower quantities in our modern diet.
The Bisgysinate Difference
The term”bisglycinate” indicates that there are two (bi-) glycine (-glycinate) molecules attached to the mineral. A glycine molecule is bound to the two valence electrons of certain minerals. Copper, iron, boron, zinc, and magnesium are minerals available as bisglycinate chelates.
While the exact mechanism of absorption is not fully understood, research has shown that chelated minerals are more bioavailable than inorganic mineral salts across many different animal species (5, 6, 7).
Bivalent metals like copper, zinc, calcium, and magnesium have a net 2+ charge – they’ve donated two electrons. Glycine’s carboxyl and amino groups are able to form a covalent bond with the metal by “sharing” an electron to fill these 2+ spaces. (8).
Some people have debated whether a chelate such as copper bysglycinate is “copper 2+” or “copper 0/neutral.” Per the science of redox reactions, when bound in the bisglycinate complex, copper in this state would technically be considered “neutral” because the charge is neutralized by sharing of electrons. If the glycine molecules are removed, it returns to Cu2+.
Studies hypothesize that the bisglycinate chelated minerals are more bioavailable because the covalent bond prevents the metals from participating in redox reactions and inhibits interactions with dietary inhibitors like phytates, saponins, and tannins (8).
Likely, the most influential impact on bioavailability is the mechanism of cellular absorption. It is suggested that amino acids chelated minerals are able to enter cells via amino acid transport systems, effectively bypassing the mineral absorption systems (3). While this has yet to be fully elucidated, it is known that amino acids enhance mineral absorption by intestinal cells (groper).
The same mechanism likely enhances cellular uptake in tissues. Glycine is a universal amino acid utilized by nearly every type of cell for various metabolic tasks (9). It’s vital in the immune system, neurological regulation, glutathione synthesis, collagen structure, and DNA and RNA synthesis. Because all tissues and cells readily absorb glycine, bisglycinate chelated minerals can more efficiently enter these cells to serve their role as vital cofactors.
It is for these reasons that Formula IQ uses bisglycinate chelated minerals in our formulas. We believe this enhances absorption and utilization of these vital minerals.
Sources:
Citation 3: Gropper SAS, Smith JL, Carr TP. Advanced Nutrition and Human Metabolism. Wadsworth; 2021.

